Carbon monoxide (CO) Catalyst Filters
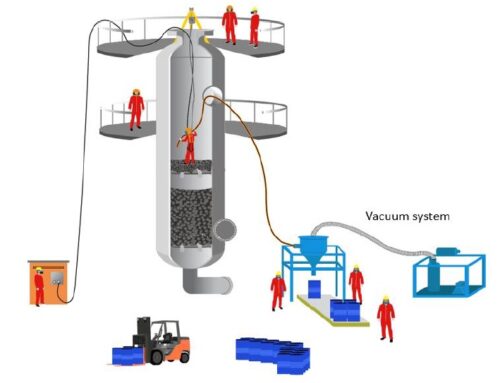
Catalyst Filters. There are applications in which the possibility of contamination by hydrocarbons (HC) or carbon monoxide (CO) is high. In these cases, one of the solutions found is the use of filters that work by catalysis.
Catalyze by gold substrate
Gold nanoparticles are very efficient in catalysis reactions at low temperatures. [Haruta et al., Chem. Lett. (1987)].
2 CO + O2 → 2 CO2
When carbon monoxide comes in contact with oxygen, on the surface of the gold nanoparticle substrate, they react according to the above reaction. In this way, carbon monoxide is transformed into CO2 which is much less toxic and dangerous. Car catalysts work in a similar way. The efficiency of catalysts, in theory, should be permanent. However, water vapors and other alkaline and acid compounds that end up reacting on the substrate make the efficiency and useful life of these filters low and need to be replaced periodically.
There are applications in which the possibility of contamination by hydrocarbons (HC) or carbon monoxide (CO) is high. In these cases, one of the solutions found is the use of filters that work by catalysis.
Catalyst Filters Catalyze by gold substrate
Gold nanoparticles are very efficient in catalysis reactions at low temperatures. [Haruta et al., Chem. Lett. (1987)].
2 CO + O2 → 2 CO2
When carbon monoxide comes in contact with oxygen, on the surface of the gold nanoparticle substrate, they react according to the above reaction. In this way, carbon monoxide is transformed into CO2 which is much less toxic and dangerous. Car catalysts work in a similar way. The efficiency of catalysts, in theory, should be permanent. However, water vapors and other alkaline and acid compounds that end up reacting on the substrate make the efficiency and useful life of these filters low and need to be replaced periodically.
There are applications in which the possibility of contamination by hydrocarbons (HC) or carbon monoxide (CO) is high. In these cases, one of the solutions found is the use of filters that work by catalysis.
Catalyst Filters Catalyze by gold substrate
Gold nanoparticles are very efficient in catalysis reactions at low temperatures. [Haruta et al., Chem. Lett. (1987)].
2 CO + O2 → 2 CO2
When carbon monoxide comes in contact with oxygen, on the surface of the gold nanoparticle substrate, they react according to the above reaction. In this way, carbon monoxide is transformed into CO2 which is much less toxic and dangerous. Car catalysts work in a similar way. The efficiency of catalysts, in theory, should be permanent. However, water vapors and other alkaline and acid compounds that end up reacting on the substrate make the efficiency and useful life of these filters low and need to be replaced periodically.