Density of Toxic Gases
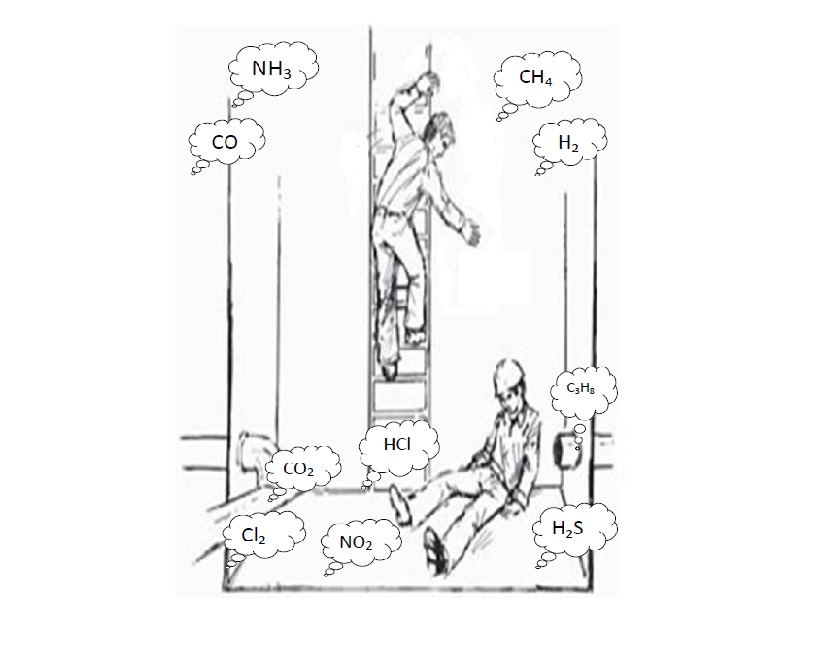
Density of Toxic Gases. The works carried out in confined space present some interesting nuances when taken to the letter in certain situations (click HERE to know what Confined Spaces are). When, for example, it is said that it is necessary to carry out a quantitative measurement of the concentrations of gases present in certain confined atmospheres before carrying out an activity with human occupation, it is not said at what depth it is necessary that this measurement be made.
Another example is when it is necessary to install fixed gas monitoring points in industrial plants. Several times I found gas detectors installed in places that are not strategic or have no functionality. With a little knowledge, it is possible to significantly reduce this gap and make better use of portable gas monitors and install fixed detectors more profitably.
The two examples, mentioned above, have in common a physical characteristic of all gas, the density (d). The density can be expressed as:
d = m / V
Where:
m – Mass
v – volume
Through the Clapeyron Equation
P · V = n · R · T
Where:
P – Pressure
V – Volume
n – number of moles of gas
R – Proportionality constant (universal gas constant)
T – Temperature (in Kelvin)
As n = m / M
Substituting in the Clapeyron formula
PV = m / M x RT
PM = m / V x R x T
Therefore:
d = (P x M) / (R x T)
Thus:
d1 / d2 = M1 / M2
Or
d1,2 = M1 / M2
As atmospheric air is a mixture of several gases, the most significant part of which is composed of 78% nitrogen (N2), and 21% oxygen (O2). Disregarding the remaining 1%, composed of several other gases, and the atomic mass of nitrogen equal to 14g and the atomic mass of oxygen equal to 16g, the molar mass of N2 28g and O2 equal to 32g and considering the proportions of the gases in the air we reach something around 28.56g. As there is still 1% discarded from the calculations, we try to round the molar mass of atmospheric air to 29g.
Therefore, when the result of the molar mass of a gas is greater than 29g, we consider that gas to be heavier than atmospheric air and, therefore, found closer to the ground. When, on the other hand, the molar mass results in something less than or equal to 29g, it is said that this gas is lighter than atmospheric air and therefore tends to rise.