The risks of inhaling
The risks of inhaling mercury. The main specific considerations of mercury for the health and safety of petrochemical workers involve exposure to mercury vapor and dermal absorption of dialkyl mercury. Comprehensive health and safety plans to prevent exposure to mercury are based on a complete understanding of the concentrations of mercury and mercury compounds in process fluids. Calculations that predict the deposition of mercury in equipment can assist in the identification of hazardous mercury accumulation sites. The detection of complete mercury vapor and an electronic monitoring program and collection of periodic samples in the atmosphere of the industrial plant is necessary to confirm and quantify the risk of exposure.
When inhaled, elemental mercury is readily absorbed into the bloodstream through the lungs. Dermal absorption efficiencies for elemental mercury in steam are typically low (less than 3 percent of the absorbed dose), but should still be strictly avoided. The main inhalation scenarios are short duration (one or two work shifts), inhalation of mercury vapor with a high concentration and moderate chronic inhalation at low level concentrations. A typical situation of short duration is a welder that repairs or cuts pipe that the mercury adsorbed on the inner wall of the corrosion products pipe. Here the vapor concentrations can be very high, due to volatilization by the torch or arc. Maintenance workers, equipment repair in plant areas dedicated to such activities, even if well ventilated, can be chronically exposed and respiratory protection programs (PPR) must be observed and put into practice.
The current US limits, through the OSHA Occupational Safety and Health Administration, the permitted exposure limit (PEL) for mercury vapor is 0.1 milligrams per cubic meter (mg / m3) of air as the upper limit. Exposure of the worker to mercury vapor must not exceed this limit level. The National Institute (USA) for Occupational Safety and Health (NIOSH) has established a recommended exposure limit (REL) for mercury vapor of 0.05 mg / m3 as a time weighted average (TWA) for up to one day of 10 hour work week and 40 hour work week.
The American Conference of Governmental Industrial Hygienists (ACGIH) assigned mercury vapor a limit value (TLV) of 0.025 mg / m3 as a TWA for a normal 8-hour work day and a 40-hour work week. Mercury vapor is not classified as a human carcinogen. The minimum risk level for chronic mercury vapor inhalation is 0, 0002 mg / m3. An MRL is an estimate of human daily exposure to a dangerous substance that is likely to be without appreciable risk of adverse health effects (cancer or otherwise) over a specified period of exposure. The US EPA reference concentration (RDM) for inhalation is calculated at 0.0003 mg / m3 (TWA).
The risks of inhaling mercury – Symptoms and Illnesses Caused
Ingesting mercury is not a typical occupational hazard for workers, but ingested mercury is often an important factor contributing to the dose for those whose diets contain a high percentage of fish. The mercury compound in fish is mono methyl mercury which is bio-accumulated and concentrated in fish-eating populations. Predatory species of marine fish (shark, tuna, swordfish, barracuda) accumulate high concentrations, as do all predatory freshwater species. Mercury and its compounds are neurotoxins. Inhalation of mercury vapor, ingestion of ionic mercury or dermal absorption of mercury compounds ultimately results in neurological dysfunction. The time period between exposure and symptom display varies considerably depending on the type (mercury species absorbed) and magnitude of exposure. Chronic exposure to mercury vapor leads to psychological anomalies (excitability, memory loss, insomnia and depression) and physical symptoms (fatigue, weakness, anorexia, weight loss). Tremors can develop in more advanced cases. Impaired renal function is seen in acute and high-dose cases.
Diagnosis and analysis for detection
Blood and urine analysis are the most common diagnostic tools for the discovery and quantification of occupational exposure. Reference levels (background level for non-occupationally exposed populations) for mercury (total) in blood, urine and hair are compiled in Table 2. Assessing worker exposure requires testing mercury in blood or urine at a frequency that is dictated by exposure risks. Mercury concentrations in the blood and urine decrease with time, but not in the same way.
Quantitative assessment of workers’ exposure to mercury of occupational origin requires a distinction between dietary mercury (ingested monomethyl mercury) and absorption in the workplace (inhaled elemental mercury and absorbed dermal dialkyl mercury). To carry out the discrimination process through analytical procedures, it is important to understand the metabolic pathways and toxicokinetics to be in a position to interpret the data.
Urine analysis is a better indicator of Hg0 inhalation exposure than is total blood Hg analysis, especially for working populations that have moderate to high fish diets. Since + CH3Hg little is eliminated through urine, the concentration of Hg in the urine (normalized to creatinine) more closely correlates with inhalation exposure. Constant dose inhalation exposure rates can be quantified to some extent, but many variables must be considered for accurate estimation. Urinary levels of about 50 ug per gram of creatinine are seen after occupational (continuous) exposure to about 40 ug Hg / m3 of air. An exposure of 40 ug mercury / m3 of air will correspond to about 20-20 ug Hg / liter of blood for an individual who does not consume significant quantities of fish. At a level of urinary mercury excretion of 100 ug per gram of creatinine, there is a high probability of developing neurological signs of mercurial intoxication.
The amount of mercury in the hair mainly reflects the CH3Hg + intake as the incorporation of mercury in the hair occurs mainly through the methylated form and not through mercury ions. Since hair can absorb some mercury vapor in the workplace, the correlation between occupational exposure and hair concentration is tenuous.
First aid treatments are largely ineffective in improving the effects of mercury absorbed by the lungs. Treatment of individuals intensely exposed to high concentrations of mercury vapor requires specialists and drugs that are rarely available in close proximity to the site of exposure. Typical hospital therapies include the administration of chelating agents that include dimercaptopropane sulfonate, with DMSA and penicillamine. Dimercaprol (BAL) and EDTA are reportedly less effective in treating mercury poisoning
The risks of exposure to n-hexane
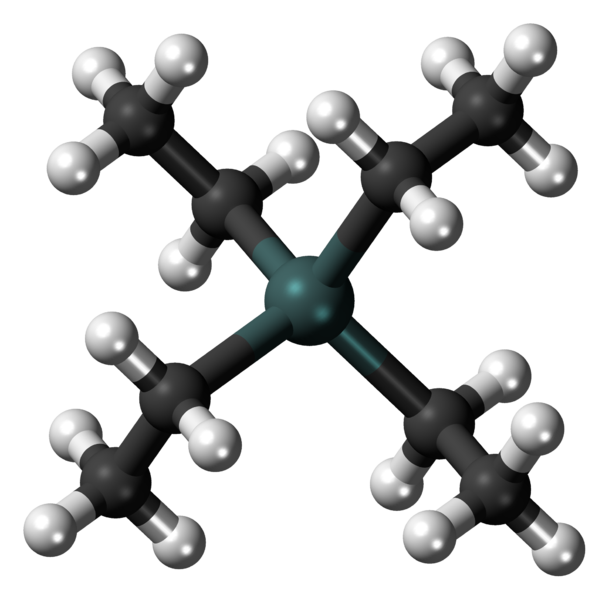
The risks of exposure to n-hexane. N-hexane is a hydrocarbon of the alkane family with the molecular formula C6H14. It is a common component of gasoline. N-hexane can be obtained by heating gasoline, obtained previously through the direct distillation of crude oil (petroleum). When gasoline is heated in the presence of a platinum catalyst, to obtain benzene and toluene, which are widely used as raw materials by the petrochemical pole industries, a by-product is n-hexane.
But the main way of obtaining the product is still the refining of crude oil. The exact composition of the fraction depends largely on the origin of the oil (crude or reformed) and the limitations of the refining process used.
Problems related to n-hexane exposure. Exposure to n-hexane can cause:
· Skin irritation (when absorbed through the skin)
· Affects the central nervous system causing decreased vision
· Decreased memory
· Mental confusion
· Dizziness
· Loss of coordination
· Headache
· Nausea
Chronic exposure can aggravate:
· Cardiovascular diseases,
· Respiratory diseases,
· Skin diseases
· Blood disorder
Companies are increasingly concerned with the occupational exposure of the workforce to risks such as n-hexane, benzene, naphtha, among others. In the past, these measures were not taken due to lack of scientific evidence or support from government agencies responsible for occupational health. Another reason was because unions, CIPAS and standards were not strong enough to promote such a campaign to combat exposure to such risks. Today, there are transmitters and detectors on the market for almost all hydrocarbon families, including benzene, toluene, xylene and n-hexane, but none of these detectors are capable of detecting a particular organic vapor in isolation.